Die Ionisierungsenergie IE oder freie Ionisierungsenthalpie (auch: Ionisationsenergie, Ionisierungspotential) bezeichnet die erforderliche Energie, um ein Elektron aus einem neutralen oder einem partiell ionisierten, gasförmigen Atom oder Molekül zu entfernen (Ionisierung):
A + IE → A+ + e-.
Die Energie für die Trennung des ersten Elektrons wird 1. Ionisierungsenergie, die für das zweite Elektron 2. Ionisierungsenergie und die für das n-te Elektron n-te Ioniserungsenergie genannt.
Ionisierungsenergien werden in eV für einzelne Elektronen oder in kJ/mol für 1 Mol = NA (Avogadro-Konstante) Elektronen (molare Ionisierungsenergie) angegeben; als Umrechnungsfaktor gilt:
1 Elektronvolt eV = 96,485307 kJ mol-1.
Bei der massenspektrometrischen Bestimmung der Ionisierungsenergien tritt bei beginnender Ionisierung ein entsprechender Massenpeak auf; in diesem Zusammenhang bezeichnet man die Ionisierungsenergie als Auftrittspotential oder Auftrittsenergie.
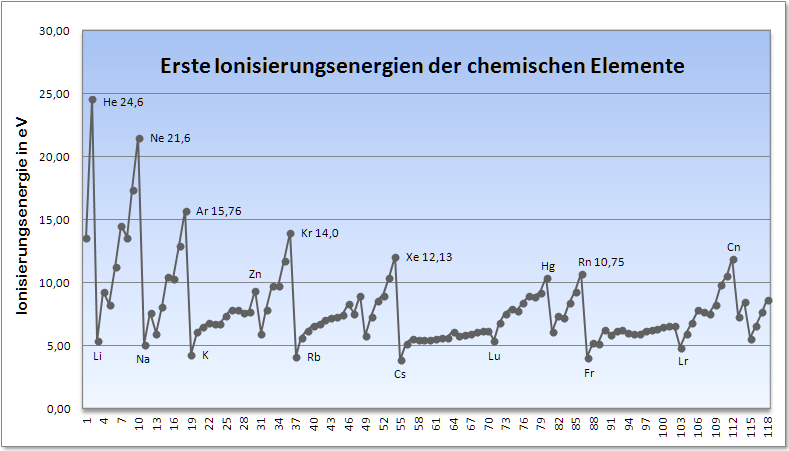
Tabelle: Ionisierungsenergien der chemischen Elemente
| OZ | E | Element | IE1 | IE2 | IE3 | IE4 | IE5 | IE6 | IE7 | IE8 |
| 1 | H | Wasserstoff | 13,598433 | - | - | - | - | - | - | - |
| 2 | He | Helium | 24,587387 | 54,41776 | - | - | - | - | - | - |
| 3 | Li | Lithium | 5,391719 | 75,64 | 122,45429 | - | - | - | - | - |
| 4 | Be | Beryllium | 9,3227 | 18,21114 | 153,89661 | 217,71865 | - | - | - | - |
| 5 | B | Bor | 8,29802 | 25,1548 | 37,93064 | 259,37521 | 340,2258 | - | - | - |
| 6 | C | Kohlenstoff | 11,2603 | 24,3833 | 47,8878 | 64,4939 | 392,087 | 489,99334 | - | - |
| 7 | N | Stickstoff | 14,5341 | 29,6013 | 47,44924 | 77,4735 | 97,8902 | 552,0718 | 667,046 | - |
| 8 | O | Sauerstoff | 13,61805 | 35,1211 | 54,9355 | 77,41353 | 113,899 | 138,1197 | 739,29 | 871,4101 |
| 9 | F | Fluor | 17,4228 | 34,9708 | 62,7084 | 87,1398 | 114,2428 | 157,1651 | 185,186 | 953,9112 |
| 10 | Ne | Neon | 21,56454 | 40,96296 | 63,45 | 97,12 | 126,21 | 157,93 | 207,2759 | 239,0989 |
| 11 | Na | Natrium | 5,1390764 | 47,2867 | 716.205 | 98,92 | 138,4 | 172,18 | 208,51 | 264,25 |
| 12 | Mg | Magnesium | 7,646235 | 15,03527 | 80,1437 | 109,2655 | 141,27 | 186,76 | 225,02 | 265,96 |
| 13 | Al | Aluminium | 5,985768 | 18,82855 | 28,44765 | 119,992 | 153,825 | 190,49 | 241,76 | 284,66 |
| 14 | Si | Silicium | 8,15168 | 16,34584 | 33,49327 | 45,14215 | 166,769 | 205,27 | 246,5 | 303,54 |
| 15 | P | Phosphor | 10,48669 | 19,7695 | 30,20288 | 51,4443 | 65,02564 | 220,423 | 263,57 | 309,6 |
| 16 | S | Schwefel | 10,36001 | 23,33788 | 34,79 | 47,222 | 72,5945 | 88,053 | 280,948 | 328,74 |
| 17 | Cl | Chlor | 12,96763 | 23,8136 | 39,61 | 53,4652 | 67,8 | 97,03 | 114,1958 | 348,28 |
| 18 | Ar | Argon | 15,75961 | 27,62966 | 40,74 | 59,81 | 75,02 | 91,009 | 124,323 | 143,46 |
| 19 | K | Kalium | 4,3406633 | 31,63 | 45,806 | 60,91 | 82,66 | 99,4 | 117,56 | 154,88 |
| 20 | Ca | Calcium | 6,11316 | 11,87172 | 50,9135 | 67,27 | 84,5 | 108,78 | 127,2 | 147,24 |
| 21 | Sc | Scandium | 6,56149 | 12,79977 | 24,75685 | 73,49 | 91,65 | 110,68 | 138 | 158,1 |
| 22 | Ti | Titan | 6,82812 | 13,5755 | 27,4919 | 43,2675 | 99,3 | 119,53 | 140,8 | 170,4 |
| 23 | V | Vanadium | 6,74619 | 14,618 | 29,311 | 46,709 | 65,2817 | 128,13 | 150,6 | 173,4 |
| 24 | Cr | Chrom | 6,76651 | 16,4857 | 30,96 | 49,16 | 69,46 | 90,6356 | 160,18 | 184,7 |
| 25 | Mn | Mangan | 7,43402 | 15,64 | 33,668 | 51,2 | 72,4 | 95,6 | 119,204 | 194,5 |
| 26 | Fe | Eisen | 7,9024 | 16,1877 | 30,652 | 54,8 | 75 | 99,1 | 124,98 | 151,06 |
| 27 | Co | Cobalt | 7,88101 | 17,084 | 33,5 | 51,3 | 79,5 | 102 | 128,9 | 157,8 |
| 28 | Ni | Nickel | 7,6398 | 18,16884 | 35,19 | 54,9 | 76,06 | 108 | 133 | 162 |
| 29 | Cu | Kupfer | 7,72638 | 20,2924 | 36,841 | 57,38 | 79,8 | 103 | 139 | 166 |
| 30 | Zn | Zink | 9,394199 | 17,96439 | 39,7233 | 59,57 | 82,6 | 108 | 134 | 174 |
| 31 | Ga | Gallium | 5,9993016 | 20,51515 | 30,7258 | 63,241 | 86,01 | 112,7 | 140,9 | 169,9 |
| 32 | Ge | Germanium | 7,89943 | 15,93461 | 34,0576 | 45,7155 | 94,5 | 115,9 | 144,9 | 176,4 |
| 33 | As | Arsen | 9,7886 | 18,5892 | 28,351 | 50,13 | 62,63 | 127,6 | - | - |
| 34 | Se | Selen | 9,75239 | 21,19 | 30,8204 | 42,945 | 68,3 | 81,7 | 155,4 | - |
| 35 | Br | Brom | 11,8138 | 21,591 | 36 | 47,3 | 59,7 | 88,6 | 103 | 192,8 |
| 36 | Kr | Krypton | 13,999605 | 24,35984 | 36,95 | 52,49 | 64,69 | 78,49 | 111 | 125,802 |
| 37 | Rb | Rubidium | 4,177128 | 27,2898 | 39 | 52,2 | 68,5 | 82,4 | 97,43 | 132,79 |
| 38 | Sr | Strontium | 5,69485 | 11,03019 | 42,88353 | 56,279 | 70,7 | 88 | 104 | 121,21 |
| 39 | Y | Yttrium | 6,2173 | 12,224 | 20,52 | 60,597 | 77 | 93 | 116 | 129 |
| 40 | Zr | Zirconium | 6,6339 | 13,13 | 22,99 | 34,34 | 80,348 | 98,46 | - | - |
| 41 | Nb | Niob | 6,75885 | 14 | 25,04 | 38,3 | 50,55 | 102,057 | 125 | - |
| 42 | Mo | Molybdän | 7,09243 | 16,16 | 27,13 | 46,4 | 54,49 | 68,8276 | 125,66 | 143,56 |
| 43 | Tc | Technetium | 7,28 | 15,26 | 29,54 | - | - | - | - | - |
| 44 | Ru | Ruthenium | 7,3605 | 16,76 | 28,47 | - | - | - | - | - |
| 45 | Rh | Rhodium | 7,4589 | 18,08 | 31,06 | - | - | - | - | - |
| 46 | Pd | Palladium | 8,3369 | 19,43 | 32,93 | - | - | - | - | - |
| 47 | Ag | Silber | 7,57623 | 21,47746 | 34,83 | - | - | - | - | - |
| 48 | Cd | Cadmium | 8,99382 | 16,90831 | 37,48 | - | - | - | - | - |
| 49 | In | Indium | 5,78636 | 18,8703 | 28,03 | 54 | - | - | - | - |
| 50 | Sn | Zinn | 7,34392 | 14,6322 | 30,5026 | 40,73502 | 72,28 | - | - | - |
| 51 | Sb | Antimon | 8,60839 | 16,63 | 25,3 | 44,2 | 56 | 108 | - | - |
| 52 | Te | Tellur | 9,0096 | 18,6 | 27,96 | 37,41 | 58,75 | 70,7 | 137 | - |
| 53 | I | Iod | 10,45126 | 19,1313 | 33 | - | - | - | - | - |
| 54 | Xe | Xenon | 12,1298 | - | - | - | - | - | - | - |
| 55 | Cs | Caesium | 3,893905 | 23,15744 | - | - | - | - | - | - |
| 56 | Ba | Barium | 5,211664 | 10,00383 | 35,844 | 47,03 | 58 | 71 | 86 | 101 |
| 57 | La | Lanthan | 5,5769 | 11,059 | 19,1774 | 49,95 | 61,6 | - | - | - |
| 58 | Ce | Cer | 5,5387 | 10,85 | 20,198 | 36,758 | 66,55 | 77,6 | - | - |
| 59 | Pr | Praseodym | 5,473 | 10,55 | 21,624 | 38,98 | 57,53 | - | - | - |
| 60 | Nd | Neodym | 5,525 | 10,72 | 22,1 | 40,4 | - | - | - | - |
| 61 | Pm | Promethium | 5,582 | 10,9 | 22,3 | 41,1 | - | - | - | - |
| 62 | Sm | Samarium | 5,6437 | 11,07 | 23,4 | 41,4 | - | - | - | - |
| 63 | Eu | Europium | 5,67038 | 11,25 | 24,92 | 42,7 | - | - | - | - |
| 64 | Gd | Gadolinium | 6,1498 | 12,09 | 20,63 | 44 | - | - | - | - |
| 65 | Tb | Terbium | 5,8638 | 11,52 | 21,91 | 39,37 | - | - | - | - |
| 66 | Dy | Dysprosium | 5,9389 | 11,67 | 22,8 | 41,4 | - | - | - | - |
| 67 | Ho | Holmium | 6,0215 | 11,8 | 22,84 | 42,5 | - | - | - | - |
| 68 | Er | Erbium | 6,1077 | 11,93 | 22,74 | 42,7 | - | - | - | - |
| 69 | Tm | Thulium | 6,18431 | 12,05 | 23,68 | 42,7 | - | - | - | - |
| 70 | Yb | Ytterbium | 6,25416 | 12,176 | 23,05 | 43,56 | - | - | - | - |
| 71 | Lu | Lutetium | 5,42586 | 13,9 | 20,9596 | 45,25 | 66,8 | - | - | - |
| 72 | Hf | Hafnium | 6,82507 | 15 | 23,3 | 33,33 | - | - | - | - |
| 73 | Ta | Tantal | 7,54957 | - | - | - | - | - | - | - |
| 74 | W | Wolfram | 7,98 | - | - | - | - | - | - | - |
| 75 | Re | Rhenium | 7,83352 | - | - | - | - | - | - | - |
| 76 | Os | Osmium | 8,43823 | - | - | - | - | - | - | - |
| 77 | Ir | Iridium | 8,96702 | - | - | - | - | - | - | - |
| 78 | Pt | Platin | 8,9588 | 18,563 | - | - | - | - | - | - |
| 79 | Au | Gold | 9,22553 | 20,2 | - | - | - | - | - | - |
| 80 | Hg | Quecksilber | 10,4375 | 18,7568 | 34,2 | - | - | - | - | - |
| 81 | Tl | Thallium | 6,108194 | 20,4283 | 29,83 | - | - | - | - | - |
| 82 | Pb | Blei | 7,41663 | 15,03248 | 31,9373 | 42,32 | 68,8 | - | - | - |
| 83 | Bi | Bismut | 7,2855 | 16,703 | 25,56 | 45,3 | 56 | 88,3 | - | - |
| 84 | Po | Polonium | 8,414 | - | - | - | - | - | - | - |
| 85 | At | Astat | 9,31751(8) | 20 | 29 | 41 | 51 | - | - | - |
| 86 | Rn | Radon | 10,7485 | - | - | - | - | - | - | - |
| 87 | Fr | Francium | 4,072741 | - | - | - | - | - | - | - |
| 88 | Ra | Radium | 5,278423 | 1.014.715 | - | - | - | - | - | - |
| 89 | Ac | Actinium | 5,17 | 11,75 | - | - | - | - | - | - |
| 90 | Th | Thorium | 6,3067 | 11,9 | 20 | 28,8 | - | - | - | - |
| 91 | Pa | Protactinium | 5,89 | - | - | - | - | - | - | - |
| 92 | U | Uran | 6,1941 | 10,6 | - | - | - | - | - | - |
| 93 | Np | Neptunium | 6,2657 | - | - | - | - | - | - | - |
| 94 | Pu | Plutonium | 6,026 | 11,2 | - | - | - | - | - | - |
| 95 | Am | Americium | 5,9738 | - | - | - | - | - | - | - |
| 96 | Cm | Curium | 5,9914 | - | - | - | - | - | - | - |
| 97 | Bk | Berkelium | 6,1979 | - | - | - | - | - | - | - |
| 98 | Cf | Californium | 6,2817 | 11,8 | - | - | - | - | - | - |
| 99 | Es | Einsteinium | 6,42 | 12 | - | - | - | - | - | - |
| 100 | Fm | Fermium | 6,5 | - | - | - | - | - | - | - |
| 101 | Md | Mendelevium | 6,58 | - | - | - | - | - | - | - |
| 102 | No | Nobelium | 6,65 | - | - | - | - | - | - | - |
| 103 | Lr | Lawrencium | 4,963(15) | 14,794 | 23,082 | 50,867 | - | - | - | - |
| 104 | Rf | Rutherfordium | 6,01 | 14,4 | 23,8 | 31,9 | - | - | - | - |
| 105 | Db | Dubnium | 6,89 | 16,03 | 24,65 | 34,19 | 44,62 | - | - | - |
| 106 | Sg | Seaborgium | 7,85 | 17,96 | 25,74 | 35,4 | 47,28 | 59,24 | - | - |
| 107 | Bh | Bohrium | 7,7 | 17,5 | 26,6 | 37,3 | 49 | 62,1 | 74,9 | - |
| 108 | Hs | Hassium | 7,6 | 18,2 | 29,3 | 37,7 | 51,2 | 64 | 78,1 | 91,8 |
| 109 | Mt | Meitnerium | 8,3 | - | - | - | - | - | - | - |
| 110 | Ds | Darmstadtium | 9,9 | - | - | - | - | - | - | - |
| 111 | Rg | Roentgenium | 10,6 | - | - | - | - | - | - | - |
| 112 | Cn | Copernicium | 11,97 | - | - | - | - | - | - | - |
| 113 | Nh | Nihonium | 7,31 | - | - | - | - | - | - | - |
| 114 | Fl | Flerovium | 8,622 | 16,576 | 34,913 | 45,584 | 60,606 | - | - | - |
| 115 | Ms | Moscovium | 5,577 | 18,234 | 27,454 | 48,485 | 59,259 | - | - | - |
| 116 | Lv | Livermorium | 6,878 | 13,779 | 29,526 | 39,472 | 62,989 | - | - | - |
| 117 | Ts | Tenness | 7,634 | 14,871 | 22,397 | 41,574 | 52,591 | - | - | - |
| 118 | Og | Oganesson | 8,888 | 16,195 | - | - | - | - | - | - |
Quellen, Referenzwerte
[1] - Jean Sansonetti: NIST Ground Levels and Ionization Energies for the Neutral Atoms.
[2] - J. E. Sansonetti, W. C. Martin, S. L. Young: NIST Handbook of Basic Atomic Spectroscopic Data.
[3] - Darleane C. Hoffman, Diana M. Lee, Valeria Pershina:
Transactinide Elements and Future Elements.
In: The Chemistry of the Actinide and Transactinide Elements, Springer, (2006), ISBN 1402035551.
Kategorie: Daten und Tabellen
Aktualisiert am 28. November 2022.
Permalink: https://www.internetchemie.info/chemie-lexikon/daten/i/ionisierungsenergie.php
© 1996 - 2026 Internetchemie ChemLin